How Neanderthals Gave Us Secret Powers
Interbreeding with our fellow hominins appears to have helped humans survive harsh climates.

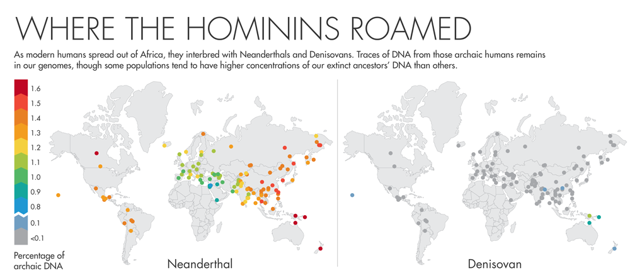
Over the last few years, scientists have dug deeper into the Neanderthal and Denisovan sections of our genomes and come to a surprising conclusion. Certain Neanderthal and Denisovan genes seem to have swept through the modern human population—one variant, for example, is present in 70 percent of Europeans—suggesting that these genes brought great advantage to their bearers and spread rapidly.
“In some spots of our genome, we are more Neanderthal than human,” said Joshua Akey, a geneticist at the University of Washington. “It seems pretty clear that at least some of the sequences we inherited from archaic hominins were adaptive, that they helped us survive and reproduce.”
“What allowed us to survive came from other species,” said Rasmus Nielsen, an evolutionary biologist at the University of California, Berkeley. “It’s not just noise, it’s a very important substantial part of who we are.”
* * *
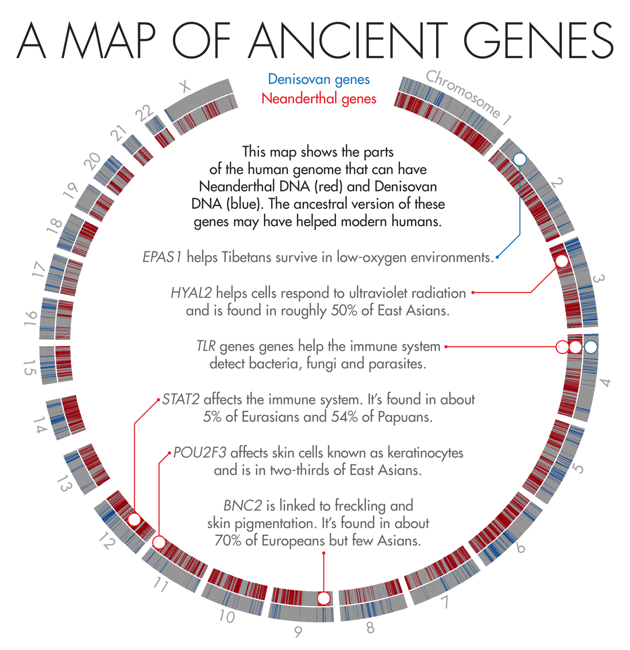
The Tibetan plateau is a vast stretch of high-altitude real estate isolated by massive mountain ranges. The scant oxygen at 14,000 feet—roughly 40 percent lower than the concentrations at sea level—makes it a harsh environment. People who move there suffer higher rates of miscarriage, blood clots, and stroke on account of the extra red blood cells their bodies produce to feed oxygen-starved tissue. Native Tibetans, however, manage just fine. Despite the meager air, they don’t make as many red blood cells as the rest of us would at those altitudes, which helps to protect their health.In 2010, scientists discovered that Tibetans owe their tolerance of low oxygen levels in part to an unusual variant in a gene known as EPAS1. About 90 percent of the Tibetan population and a smattering of Han Chinese (who share a recent ancestor with Tibetans) carry the high-altitude variant. But it’s completely absent from a database of 1,000 human genomes from other populations.
In 2014, Nielsen and colleagues found that Tibetans or their ancestors likely acquired the unusual DNA sequence from Denisovans, a group of early humans first described in 2010 that are more closely related to Neanderthals than to us. The unique gene then flourished in those who lived at high altitudes and faded away in descendants who colonized less harsh environments. “That’s one of the most clear-cut examples of how [interbreeding] can lead to adaptation,” said Sriram Sankararaman, a geneticist and computer scientist at the University of California, Los Angeles.
This phenomenon has been well documented in a number of species, including mice that adopted other species’ tolerance to pesticides and butterflies that appropriated other species’ wing patterning. But it was difficult to study adaptive introgression in humans until the first Neanderthal genome was sequenced in 2010, providing scientists with hominin DNA to compare to our own.
Neanderthals and Denisovans would have been a good source of helpful DNA for our ancestors. They had lived in Europe and Asia for hundreds of thousands of years—enough time to adjust to the cold climate, weak sun and local microbes. “What better way to quickly adapt than to pick up a gene variant from a population that had probably already been there for 300,000 years?” Akey said. Indeed, the Neanderthal and Denisovan genes with the greatest signs of selection in the modern human genome “largely have to do with how humans interact with the environment,” he said.
Scientists surmise that BNC2 and other skin genes helped modern humans adapt to northern climates, but it’s not clear exactly how. Skin can have many functions, any one of which might have been helpful. “Maybe skin pigmentation, or wound healing, or pathogen defense, or how much water loss you have in an environment, making you more or less susceptible to dehydration,” Akey said. “So many potential things could be driving this—we don’t know what differences were most important.”
* * *
One of the deadliest foes that modern humans had to fight as they ventured into new territories was also the smallest—novel infectious diseases for which they had no immunity. “Pathogens are one of the strongest selective forces out there,” said Janet Kelso, a bioinformatician at the Max Planck Institute for Evolutionary Anthropology in Leipzig, Germany.Earlier this year, Kelso and collaborators identified a large stretch of Neanderthal DNA—143,000 DNA base-pairs long—that may have played a key role in helping modern humans fight off disease. The region spans three different genes that are part of the innate immune system, a molecular surveillance system that forms the first line of defense against pathogens. These genes produce proteins called toll-like receptors, which help immune cells detect foreign invaders and trigger the immune system to attack.
Kelso speculates that this variant might have boosted early humans’ resistance to different kinds of bacteria. That would have helped modern humans as they colonized new territories. Yet this added resistance came at a price. “The trade-off for that was a more sensitive immune system that was more sensitive to nonpathogenic allergens,” said Kelso. But she was careful to point out that this is just a theory. “At this point, we can hypothesize a lot, but we don’t know exactly how this is working.”
Most of the Neanderthal and Denisovan genes found in the modern genome are more mysterious. Scientists have only a vague idea of what these genes do, let alone how the Neanderthal or Denisovan version might have helped our ancestors. “It’s important to understand the biology of these genes better, to understand what selective pressures were driving the changes we see in present-day populations,” Akey said.
A number of studies like Kelso’s are now under way, trying to link Neanderthal and Denisovan variants frequently found in contemporary humans with specific traits, such as body-fat distribution, metabolism or other factors. One study of roughly 28,000 people of European descent, published in Science in February, matched archaic gene variants with data from electronic health records. Overall, Neanderthal variants are linked to higher risk of neurological and psychiatric disorders and lower risk of digestive problems. (That study didn’t focus on adaptive DNA, so it’s unclear how the segments of archaic DNA that show signs of selection affect us today.)
At present, much of the data available for such studies is weighted toward medical problems—most of these databases were designed to find genes linked to diseases such as diabetes or schizophrenia. But a few, such as the U.K. Biobank, are much broader, storing information on participants’ vision, cognitive test scores, mental health assessments, lung capacity and fitness. Direct-to-consumer genetics companies also have large, diverse data sets. For example, 23andMe analyzes users’ genetics for clues about ancestry, health risk and other sometimes bizarre traits, such as whether they have a sweet tooth or a unibrow.
Of course, not all the DNA we got from Neanderthals and Denisovans was good. The majority was probably detrimental. Indeed, we tend to have less Neanderthal DNA near genes, suggesting that it was weeded out by natural selection over time. Researchers are very interested in these parts of our genomes where archaic DNA is conspicuously absent. “There are some really big places in the genome with no Neanderthal or Denisovan ancestry as far as we can see—some process is purging the archaic material from these regions,” Sankararaman said. “Perhaps they are functionally important for modern humans.”
This post appears courtesy of Quanta Magazine.

No comments:
Post a Comment