At Memorial Sloan Kettering, we believe that immunotherapy is one of the most promising ways to treat, cure, and ultimately prevent cancer.
Immunotherapy was born at MSK more than a century ago. Since then our scientists have led the effort to develop new immune-based treatments for cancer. Our researchers have been at the epicenter of new discoveries in the field, and their work is bringing exciting new treatment options to people with cancer.
Patients who come to MSK for immunotherapy treatment benefit from unparalleled expertise in a field that our scientists pioneered.



How We Care for Patients
- Our immunotherapy patients benefit from the close collaboration between our doctors and scientists.
- Discoveries in the lab are constantly being translated into new therapies for patients.
- We’re currently running nearly 100 immunotherapy-focused clinical trials.
- Our experts are seeking out new ways to help the immune system recover after a bone marrow transplant.
- Our science has already begun to change how melanoma, leukemia, and lung, bladder, and kidney cancers are treated.
MSK is home to a diverse group of scientists who study the immune system in all its complexity. Our laboratory scientists include many immunologists, geneticists, and cell biologists who explore the biology of immune cells and their interactions with tumors and infectious organisms.
Our physician-scientists are developing groundbreaking immune therapies that are helping to treat several forms of advanced cancer. They have played a lead role in developing and testing the immunotherapy drugs known ascheckpoint inhibitors that “release the brakes” on the immune system, allowing it to mount a stronger attack against cancer. These drugs are transforming the way many cancers are treated, among them melanoma, lung, bladder, and kidney cancers.
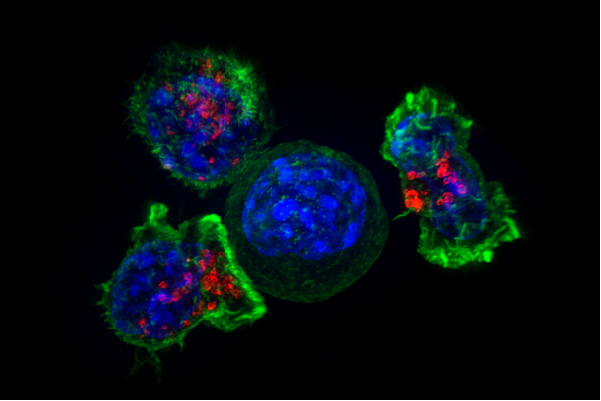
MSK scientists have also led the way in using chimeric antigen receptor (CAR) T cell therapy to treat leukemia and certain solid tumors. In this approach, immune cells from a patient are removed from the body, armed with new proteins that recognize cancer, and given back to the patient in large numbers.
Where Immunotherapy Began
Memorial Sloan Kettering’s roots in immunotherapy extend all the way back to 1893, when bone surgeon and cancer researcher William Coley began his work here on bacterial vaccines.
In the decades that followed, MSK scientists put cancer immunotherapy on firm scientific footing. Their many discoveries have helped make immunotherapy what it is today.
View our timeline of progress to see how MSK scientists have been at the forefront of immunotherapy research for more than 120 years.
Parker Institute for Cancer Immunotherapy
Immunity science at MSK spans one academic program and four collaborative research centers, including our newest center, the Parker Institute for Cancer Immunotherapy. The Parker Institute, established by tech entrepreneur Sean Parker, brings together top researchers in the field from Memorial Sloan Kettering and five other founding partner institutions, with the aim of speeding the discovery and development of new immunotherapy treatment options.
MSK’s Parker Institute members are dreaming big — pursuing projects that will not only improve existing immunotherapies but also open the door to new ones. The support offered through the Parker Institute will allow them to build on MSK’s leadership as we search for new ways to extend the benefits of immunotherapy to even more patients.
Immunotherapy is the disruptive technology of cancer medicine.
Parker Institute Director Jedd Wolchok has played a central role in developing and testing the immunotherapy drugs known as checkpoint inhibitors. He led pivotal clinical trials leading to the FDA approval of ipilimumab, the first immune checkpoint inhibitor, for advanced melanoma in 2011. He also led studies leading to the FDA approval of the first-ever immunotherapy combination for people with advanced melanoma.
Co-Director Marcel van den Brink is a world-renowned expert on cell-based immunotherapy and bone marrow transplantation, approaches that are successfully treating and in some cases curing several types of blood cancer. His work has led to novel therapeutic strategies for graft-versus-host disease and immune reconstitution after bone marrow transplantation.



























No comments:
Post a Comment